Abstract
Background: CD19 CAR T cell therapies have demonstrated high initial complete remission (CR) rates of 70-80% in relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) with durable remissions observed in a subset of patients. Despite the remarkable initial CR rate, relapses occur in 20-45% of patients and with increasing use of CD19 CAR T cell therapies in ALL, a key remaining clinical question is the role of post-CAR HCT.
Methods: Patients up to 26 years treated with the commercial (i.e., tisagenlecleucel) or investigational CD19 CAR T cell products from 2014 to 2019 in the United States were included. The objectives were to examine the impact of post-CAR HCT on mortality, disease-free survival (DFS), leukemia relapse, GVHD and transplant related mortality among HCT recipients. The intent of HCT was analyzed as consolidation when there was no evidence of post-CAR T cell relapse prior to HCT. Cox regression multivariable analysis used HCT as time-dependent variable along with age, performance status and time from diagnosis to CAR-T cell infusion to evaluate its impact on outcomes.
Results: We identified 347 patients who received CD19 CAR T cells from 86 centers. Median age was 13 (range, 0.4-26 years) with 39% of patients from Hispanic or Latino ethnic origin. Patients were high risk and heavily pretreated; 56% had poor cytogenetics, 51% had ≥3 line of prior therapies and 34% had prior HCT. Most of the patients (74%) received tisagenlecleucel. Disease burden at the time of CAR T cell infusion included morphologic disease (≥5% bone marrow blasts) (65%), measurable residual disease (MRD) positive (16%) and negative CR (18%). CRS of all grades was observed in 57.6% and grade 3-4 CRS in 13%; neurotoxicity of all grades was observed in 25% and grade 3-4 in 8%. With a median follow-up of 12.7 months (range, 2.2-60.3), DFS at 3, 6 and 12 months following CAR T cell infusion was 80.9% % (95% CI: 76.6%-84.9%), 71.2% (95% CI: 66.1%-76.0%) and 57.6% (95% CI: 51.8%-63.3%), respectively. OS at 3, 6 and 12 months was 93.6% (95% CI: 90.8%-96.0%), 89.8% (95% CI: 86.4%-92.8%) and 79.4% (95% CI: 74.7%-83.6%), respectively. Incidences of relapse without censoring at subsequent HCT at month 3, 6 and 12 were 18.5% (95% CI: 14.5%-22.8%), 28.2% (95% CI: 23.5%-33.3%), and 40.6% (95% CI: 35.0%-46.3%), respectively.
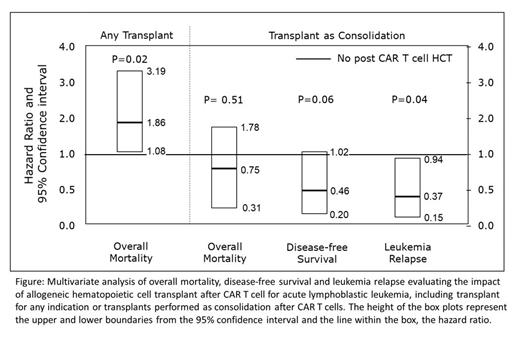
Of the 347 patients who received CD19 CAR therapy, 62 patients (18%) had subsequent HCT in CR as a consolidation post-CAR with a median time from CAR T to HCT of 4.7 months (range, 1.1-18.6). 36% had a prior HCT (i.e., pre-CAR), 87% received myeloablative regimen and among the 57 patients with MRD evaluation 56 were negative (98%). Frequencies of post-CAR HCT did not change during the 5-year study period. Overall, transplant related mortality (TRM) at month 6 was 8.9% (95% CI: 3.6-16.1), and grade 2-4 acute GvHD was observed in 38.0% (95% CI: 28.4-48.2) of patients at month 6. Post-CAR HCT used as a consolidation was associated with lower relapse (hazard ratio (HR) 0.37; p=0.037) but no significant impact on mortality (HR 0.75, p=0.51) or DFS (HR=0.46, p=0.057). When accounting for any HCT performed after CAR T cell, HCT was associated with higher mortality (Figure).
Conclusions: This is the largest clinical series to date examining the impact of post-CAR HCT on clinical outcome of R/R B-ALL patients. We found that 18% of the patients had post-CAR HCT as a consolidation therapy with a favorable safety profile of 8.9% TRM rate despite a prior transplant rate of 36%. Consolidation HCT after CAR T cell resulted in significant reduction in leukemia relapse with a trend towards better DFS. Consolidation HCT seem safe and incurred a benefit by reducing disease relapse. Further studies in understanding the role of consolidation HCT as planned, or post-CAR T cell response-based strategy or treatment for early B-cell recovery are needed to further define the best candidates for HCT after tisagenlecleucel .
Park: BMS: Consultancy; Affyimmune: Consultancy; Kite Pharma: Consultancy; Curocel: Consultancy; Novartis: Consultancy; Minerva: Consultancy; Innate Pharma: Consultancy; PrecisionBio: Consultancy; Intellia: Consultancy; Amgen: Consultancy; Autolus: Consultancy; Servier: Consultancy; Artiva: Consultancy; Kura Oncology: Consultancy. Nikiforow: Kite/Gilead: Other: ad HOC Advisory Boards; Novartis: Other: ad Hoc Advisory Boards; Iovance: Other: ad Hoc Advisory Boards; Glaxo Smith Kline (GSK): Other: ad Hoc Advisory Boards. Hu: Kite/Gilead: Research Funding; Novartis: Research Funding; Celgene: Research Funding. Ahmed: Merck: Research Funding; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seagen: Research Funding; Xencor: Research Funding. Badar: Pfizer Hematology-Oncology: Membership on an entity's Board of Directors or advisory committees. Cairo: Servier: Speakers Bureau; Sanofi: Speakers Bureau; Amgen: Speakers Bureau; Jazz Pharmaceutical: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Speakers Bureau; Omeros: Membership on an entity's Board of Directors or advisory committees; Nektar: Membership on an entity's Board of Directors or advisory committees. Dholaria: Janssen: Research Funding; Takeda: Research Funding; Jazz: Speakers Bureau; MEI: Research Funding; Pfizer: Research Funding; Angiocrine: Research Funding; Poseida: Research Funding; Celgene: Speakers Bureau. Grover: Kite: Other: Advisory Board; Genentech: Research Funding; ADC: Other: Advisory Board; Tessa: Consultancy; Novartis: Consultancy. Locke: Gerson Lehrman Group: Consultancy; Emerging Therapy Solutions: Consultancy; Moffitt Cancer Center: Patents & Royalties: field of cellular immunotherapy; Calibr: Consultancy, Other: Scientific Advisory Role; BMS/Celgene: Consultancy, Other: Scientific Advisory Role; Wugen: Consultancy, Other; Legend Biotech: Consultancy, Other; Janssen: Consultancy, Other: Scientific Advisory Role; Iovance Biotherapeutics: Consultancy, Other: Scientific Advisory Role; Novartis: Consultancy, Other, Research Funding; GammaDelta Therapeutics: Consultancy, Other: Scientific Advisory Role; Takeda: Consultancy, Other; EcoR1: Consultancy; Cowen: Consultancy; Umoja: Consultancy, Other; Kite, a Gilead Company: Consultancy, Other: Scientific Advisory Role, Research Funding; Cellular Biomedicine Group: Consultancy, Other: Scientific Advisory Role; Bluebird Bio: Consultancy, Other: Scientific Advisory Role; Amgen: Consultancy, Other: Scientific Advisory Role; Allogene Therapeutics: Consultancy, Other: Scientific Advisory Role, Research Funding. Murthy: CRISPR Therapeutics: Research Funding. Mussetti: GILEAD: Other: Clinical trials participation, Research Funding; TAKEDA: Honoraria; NOVARTIS: Honoraria, Other: Clinical trials participation. Pulsipher: Jasper Therapeutics: Honoraria; Adaptive: Research Funding; Equillium: Membership on an entity's Board of Directors or advisory committees. Qayed: Novartis: Membership on an entity's Board of Directors or advisory committees; Medexus: Membership on an entity's Board of Directors or advisory committees; Mesoblast: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; BMS: Other. Reshef: Gilead, BMS, Precision, Immatics, Atara, Takeda, Shire, Pharmacyclics, Incyte: Research Funding; Gilead and Novartis: Honoraria; Bayer, BMS, Regeneron, TScan, Synthekine, Atara, Jasper: Consultancy. Rizzieri: Kite: Honoraria, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Acrotech: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; AROG: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Celltrion: Membership on an entity's Board of Directors or advisory committees; Mustang: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria; Pharmacyclics: Honoraria. Sharma: Vertex Pharmaceuticals/CRISPR Therapeutics: Other: Salary support paid to institution; Medexus Inc: Consultancy; CRISPR Therapeutics: Other, Research Funding; Vindico Medical Education: Honoraria; Spotlight Therapeutics: Consultancy; Novartis: Other: Salary support paid to institution. Schultz: BMS DSMB: Other: payment; Sobi: Membership on an entity's Board of Directors or advisory committees. Turtle: Century Therapeutics Ad hoc advisory boards (last 12 months): Nektar Therapeutics, Allogene, PACT Pharma, Astra Zeneca, Amgen: Membership on an entity's Board of Directors or advisory committees; Precision Biosciences: Membership on an entity's Board of Directors or advisory committees; Eureka Therapeutics: Membership on an entity's Board of Directors or advisory committees; Caribou Biosciences: Membership on an entity's Board of Directors or advisory committees; T-CURX: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Membership on an entity's Board of Directors or advisory committees; ArsenalBio: Membership on an entity's Board of Directors or advisory committees; Minerva: Other; Nektar Therapeutics: Other; Juno Therapeutics/BMS: Other: Payments, Patents & Royalties: Patent licensed to Juno Therapeutics.; Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, ArsenalBio: Current holder of stock options in a privately-held company. Pasquini: Kite Pharma: Research Funding; Novartis: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; GlaxoSmithKline: Research Funding. Perales: MorphoSys: Honoraria; Cidara: Honoraria; Bristol-Myers Squibb: Honoraria; Miltenyi Biotec: Honoraria, Other; Sellas Life Sciences: Honoraria; Takeda: Honoraria; Karyopharm: Honoraria; Celgene: Honoraria; Omeros: Honoraria; Nektar Therapeutics: Honoraria, Other; Equilium: Honoraria; Novartis: Honoraria, Other; NexImmune: Honoraria; Incyte: Honoraria, Other; Kite/Gilead: Honoraria, Other; Merck: Honoraria; Servier: Honoraria; Medigene: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal